The Shapes of Molecules
While Lewis dot structures show how atoms are bonded in a molecule, they do not reveal the molecule’s shape. To predict molecular shape, we use the VSEPR (Valence Shell Electron Pair Repulsion) model. The key idea is that molecules adopt shapes that minimize repulsions between electron pairs.
When minimizing electron pair repulsions, two types of electron sets must be considered:
- Bonding pairs: Electrons involved in single or multiple covalent bonds.
- Non-bonding pairs: Lone pairs localized on a single atom.
The VSEPR Model – Determining Molecular Shape
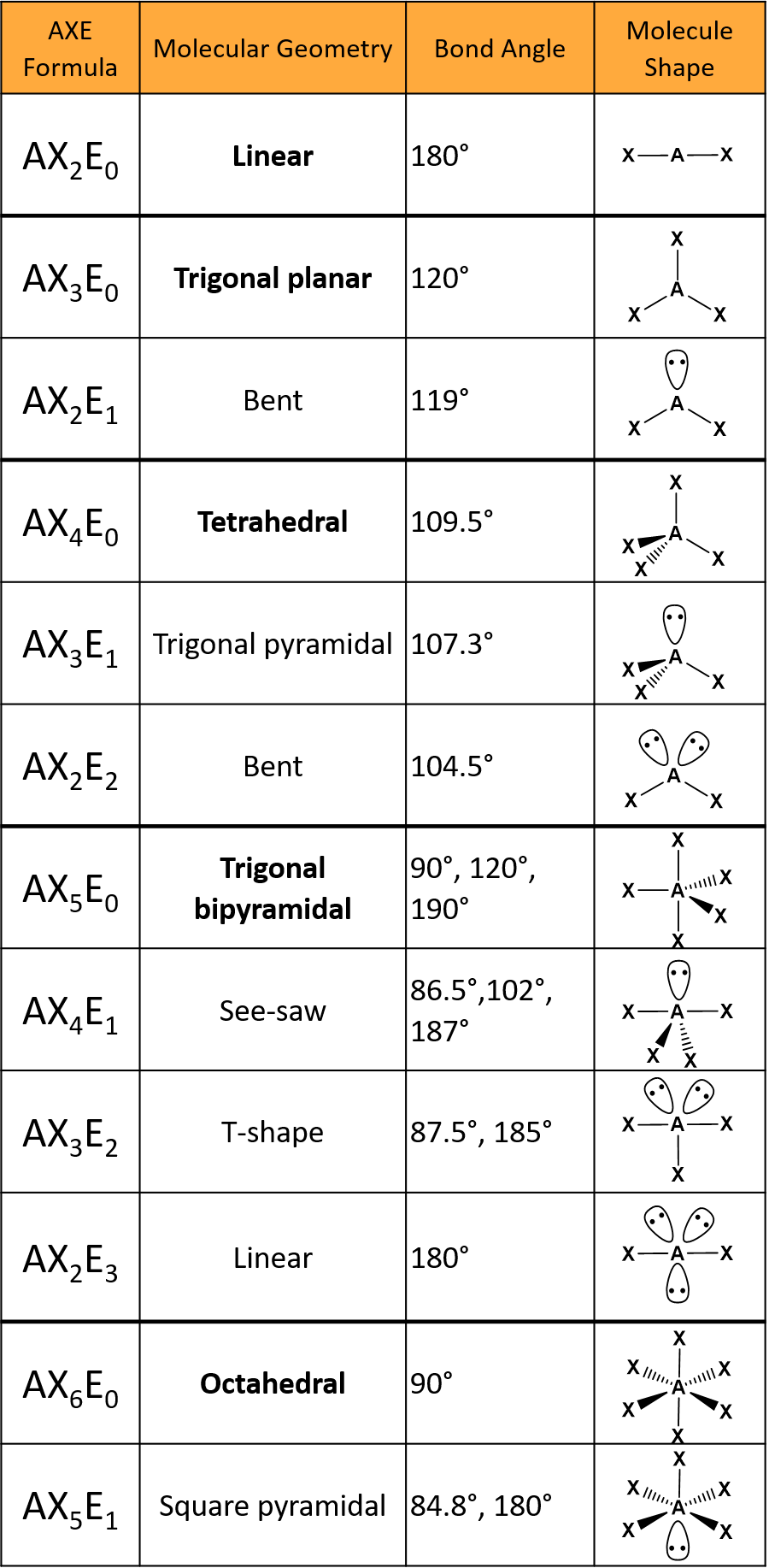
The shape of a molecule is determined by the number of bonding and non-bonding electron pairs around the central atom. Here is a guide:
| Total Number of Bonds and Lone Pairs | Structural Pair Geometry | Shape |
|---|---|---|
| 2 | Linear | Linear |
| 3 | Trigonal Planar | Trigonal Planar |
| 4 | Tetrahedral | Tetrahedral |
| 5 | Trigonal Bipyramidal | Trigonal Bipyramidal |
| 6 | Octahedral | Octahedral |
 Credit: Expii
Credit: Expii
Follow these steps to determine molecular geometry:
- Draw the Lewis structure and count single bonds, multiple bonds, and lone pairs.
- Arrange the electron pairs to minimize repulsion based on the VSEPR model.
- Determine the molecular geometry using the table above.
Example: Methane (CH4)
In methane, there are four bonding pairs and no lone pairs. The shape is tetrahedral. Lone pairs, when present, exert greater repulsive force than bonding pairs, causing bond angles to adjust.
Expanded Octet and Molecular Geometry
Only elements from the third period and beyond can expand their octet, using d orbitals to bond with five or six atoms. For example, in XeF4, xenon has two lone pairs and four bonding pairs.
There are two possible arrangements:
- Axial arrangement: Shared pairs are "top and bottom."
- Equatorial arrangement: Lone pairs are 180° apart, minimizing repulsion and providing greater stability.
In both arrangements, the electronic geometry is octahedral (90° bond angles). The seesaw shape comes from axial lone pairs, while the square planar shape results from equatorial lone pairs, which is more stable.
Example: SeF4 Hybridization
To determine the hybridization of selenium in SeF4:
- Valence electrons: Se (6) + 4 × F (7) = 34 electrons, or 17 pairs.
- Se is bonded to four fluorines and has one lone pair, totaling five areas of electron density.
- This corresponds to sp3d hybridization.
Quick Summary of Electron Density and Shapes
- 2 areas: Linear
- 3 areas: Trigonal planar
- 4 areas: Tetrahedral
- 5 areas: Trigonal bipyramidal
- 6 areas: Octahedral
Molecular Polarity
Polarity in chemical bonds occurs when electrons are unevenly shared between two atoms, creating slight charges on each side. Molecules with positive and negative ends are called dipoles.
For example, in water (H2O), the oxygen atom, with its two lone pairs, creates a negative pole, while the hydrogen atoms create a positive pole. This polarity explains many of water’s special properties.
However, not all molecules with polar bonds are polar overall. In carbon dioxide (CO2), although each C—O bond is polar, the molecule is linear, and the bond polarities cancel out, making CO2 nonpolar.